22.1 Lifespan, Life Expectancy, & Healthy Aging
Learning Objectives
- Explain the difference between life expectancy and lifespan.
Life Expectancy vs. Lifespan
Lifespan or maximum lifespan refer to the greatest age reached by any member of a given species (or population). For humans, the lifespan is currently between 120 and 125. Life expectancy is defined as the average number of years that members of a species (or population) live. According to the World Health Organization (WHO) (2019) global life expectancy for those born in 2019 is 72.0 years, with females reaching 74.2 years and males reaching 69.8 years. Women live longer than men around the world, and the gap between the sexes has remained the same since 1990. Overall life expectancy ranges from 61.2 years in the WHO African Region to 77.5 years in the WHO European Region. Global life expectancy increased by 5.5 years between 2000 and 2016. Improvements in child survival and access to antiretroviral medication for the treatment of HIV are considered the main reasons for this increase. However, life expectancy in low-income countries (62.7 years) is 18.1 years lower than in high-income countries (80.8 years). In high-income countries, the majority of people who die are old, while in low-income countries almost one in three deaths are in children under 5 years of age. According to the Central Intelligence Agency (2019) the United States ranks 45th in the world for life expectancy.
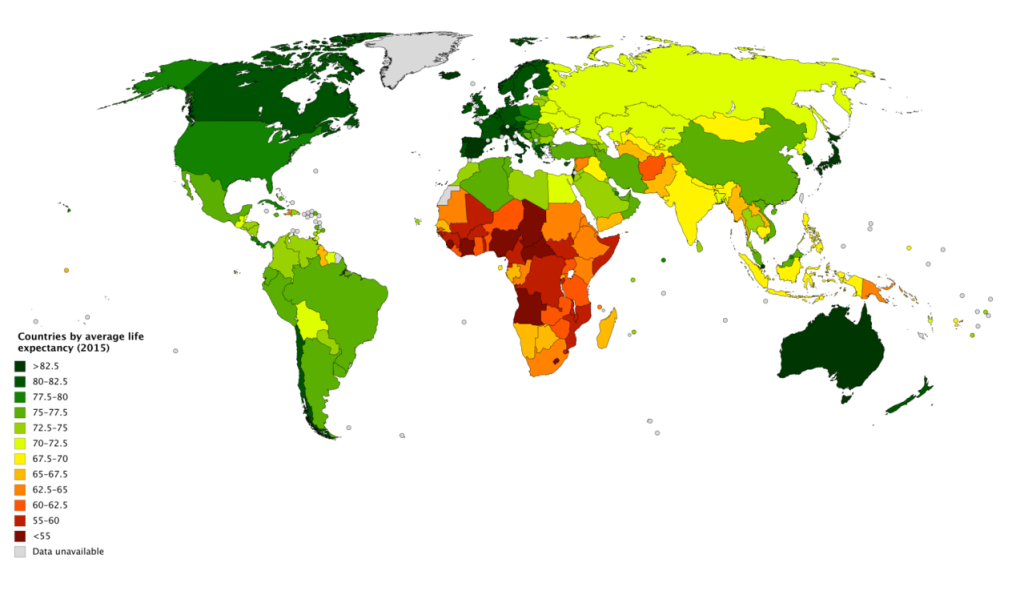
World Healthy Life Expectancy
A better way to appreciate the diversity of people in late adulthood is to go beyond chronological age and examine how well the person is aging. Many in late adulthood enjoy better than average health and social well-being and so are aging at an optimal level. In contrast, others experience poor health and dependence to a greater extent than would be considered typical. When looking at large populations, the WHO (2019) measures how many equivalent years of full health on average a newborn baby is expected to have. This age, called The Healthy Life Expectancy, takes into account current age-specific mortality, morbidity, and disability risks. In 2016, the global Healthy Life Expectancy was 63.3 years up from 58.5 years in 2000. The WHO African Region had the lowest Healthy Life Expectancy at 53.8 years, while the WHO Western Pacific Region had the highest at 68.9 years.
Life Expectancy in America
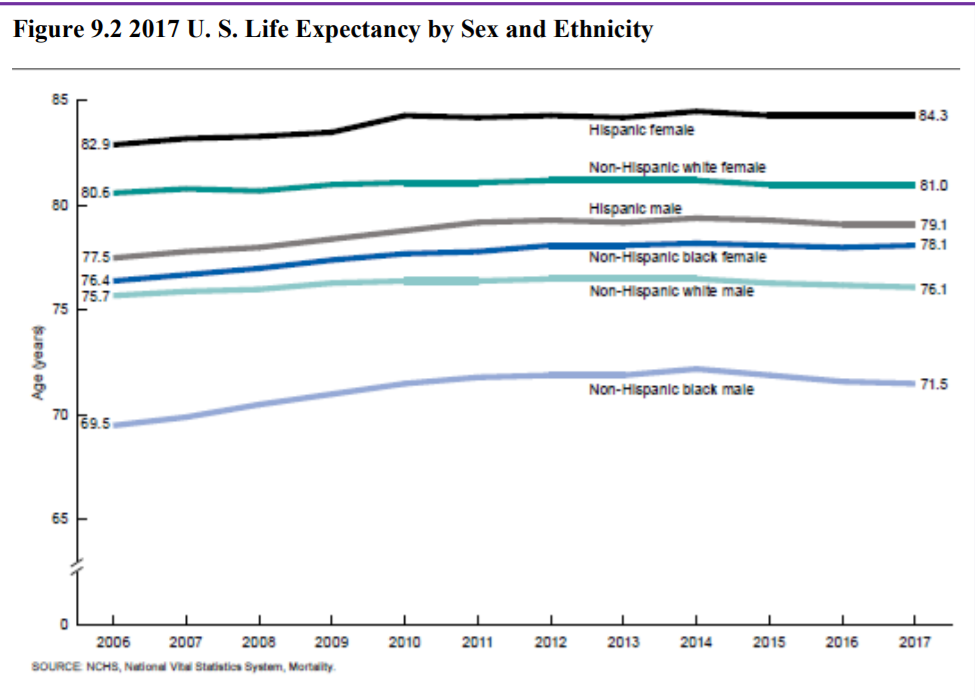
The overall life expectancy for a baby born in 2017 in the United States is 78.6 years, decreasing from 78.7 years in 2016 and 78.8 years in 2015 (Arias & Xu, 2019). The decrease from 2016 occurred for males, changing from 76.2 years to 76.1 years, while it did not change for females (81.1 years). Life expectancy at birth decreased by 0.1 year for the non-Hispanic white population (78.6 to 78.5). Life expectancy at birth did not change from 2016 for the non-Hispanic black population (74.9), and the Hispanic population (81.8). Before this unprecedented two-year decline, life expectancy had been increasing steadily for decades. Reasons given by the CDC for this decrease in life expectancy include deaths from drug overdoses, an increase in liver disease, and a rise in suicide rates (Saiidi, 2019). Figure 10.18 shows the United States life expectancy from 2006-2017 by ethnicity and sex.
American Healthy Life Expectancy
To determine the current United States Healthy Life Expectancy (HLE), factors were evaluated in 2007-2009 to determine how long an individual currently at age 65 will continue to experience good health (CDC, 2013). The highest Healthy Life Expectancy (HLE) was observed in Hawaii with 16.2 years of additional good health, and the lowest was in Mississippi with only 10.8 years of additional good health. Overall, the lowest HLE was among southern states. Females had a greater HLE than males at age 65 years in every state and DC. HLE was greater for whites than for Blacks in DC and all states from which data were available, except in Nevada and New Mexico.
Link to Learning: Blue Zones
Recent research on longevity reveals that people in some regions of the world live significantly longer than people elsewhere. Efforts to study the common factors between these areas and the people who live there is known as blue zone research. Blue zones are regions of the world where Dan Buettner claims people live much longer than average. The term first appeared in his November 2005 National Geographic magazine cover story, “The Secrets of a Long Life.” Buettner identified five regions as “Blue Zones”: Okinawa (Japan); Sardinia (Italy); Nicoya (Costa Rica); Icaria (Greece); and the Seventh-day Adventists in Loma Linda, California. He offers an explanation, based on data and first hand observations, for why these populations live healthier and longer lives than others.
The people inhabiting blue zones share common lifestyle characteristics that contribute to their longevity. The Venn diagram below (Figure 10.19) highlights the following six shared characteristics among the people of Okinawa, Sardinia, and Loma Linda blue zones. Though not a lifestyle choice, they also live as isolated populations with a related gene pool.
- Family – put ahead of other concerns
- Less smoking
- Semi-vegetarianism – the majority of food consumed is derived from plants
- Legumes commonly consumed
- Constant moderate physical activity – an inseparable part of life
- Social engagement – people of all ages are socially active and integrated into their communities
In his book, Buettner provides a list of nine lessons, covering the lifestyle of blue zones people:
- Moderate, regular physical activity.
- Life purpose.
- Stress reduction.
- Moderate caloric intake.
- Plant-based diet.
- Moderate alcohol intake, especially wine.
- Engagement in spirituality or religion.
- Engagement in family life.
- Engagement in social life.
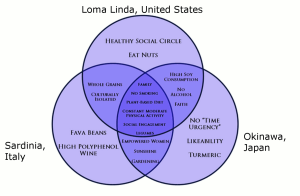
Figure 22.4. Blue zones share many common healthy habits
contributing to longer lifespans.
Although improvements have occurred in overall life expectancy, children born in America today may be the first generation to have a shorter life span than their parents. Much of this decline has been attributed to the increase in sedentary lifestyle and obesity. Since 1980, the obesity rate for children between the ages of 2 and 19 has tripled, as 20.5% of children were obese in 2014 compared with 5% in 1980 (American Medical Association, 2016). Obesity in children is associated with many health problems, including high blood pressure, type 2 diabetes, elevated blood cholesterol levels, and psychological concerns including low self-esteem, negative body image, and depression. Excess weight is associated with an earlier risk of obesity-related diseases and death. In 2007, former Surgeon General Richard Carmona stated, “Because of the increasing rates of obesity, unhealthy eating habits and physical inactivity, we may see the first generation that will be less healthy and have a shorter life expectancy than their parents” (p. 1).
Gender Differences in Life Expectancy
Several explanations have been offered for the gender differences in average life expectancy that emerged in the late 1800s– one focused on nature and the other on nurture.
Biological Explanations
Biological differences in sex chromosomes and different pattern of gene expression are theorized as one reason why females live longer (Chmielewski, Boryslawski, & Strzelec, 2016). Males are heterogametic (XY), whereas females are homogametic (XX) with respect to sex chromosomes. Males can only express the X chromosome genes that come from the mother, while females have the advantage of being able to select the “better” X chromosome from their mother or father, while inactivating the “worse” X chromosome. This process of selection for “better” genes is impossible in males and results in the greater genetic and developmental stability of females.
In terms of developmental biology, women are the “default” sex, which means that the creation of a male individual requires a sequence of events at a molecular level. According to Chmilewski et al. (2016), these events are initiated by the activity of the SRY gene located on the Y chromosome. This activity and change in the direction of development results in a greater number of disturbances and developmental disorders, because the normal course of development requires many different factors and mechanisms, each of which must work properly and at a specific stage of the development (p. 134).
Men are more likely to contract viral and bacterial infections, and their immunity at the cellular level decreases significantly faster with age. Although women are slightly more prone to autoimmune and inflammatory diseases, such as rheumatoid arthritis, the gradual deterioration of the immune system is slower in women (Caruso, Accardi, Virruso, & Candore, 2013; Hirokawa et al., 2013).
Looking at the influence of hormones, estrogen levels in women appear to have a protective effect on their heart and circulatory systems (Viña, Borrás, Gambini, Sastre, & Pallardó, 2005). Estrogens also have antioxidant properties that protect against harmful effects of free radicals, which damage cell components, cause mutations, and are in part responsible for the aging process. Testosterone levels are higher in men than in women and are related to more frequent cardiovascular and immune disorders. The level of testosterone is also responsible, in part, for male behavioral patterns, including increased level of aggression and violence (Martin, Poon, & Hagberg, 2011; Borysławski & Chmielewski, 2012).
Another factor responsible for risky behavior is the frontal lobe of the brain. The frontal lobe, which controls judgment and consideration of an action’s consequences, develops more slowly in boys and young men. This lack of judgment affects lifestyle choices, and consequently many more boys and men die by smoking, excessive drinking, accidents, drunk driving, and violence (Shmerling, 2016).
Lifestyle Factors
Certainly not all the reasons women live longer than men are biological. As previously mentioned, male behavioral patterns and lifestyle play a significant role in the shorter lifespans for males. One significant factor is that males work in more dangerous jobs, including police, fire fighters, and construction, and they are more exposed to violence. According to the Federal Bureau of Investigation (2014) there were 11,961 homicides in the U.S. in 2014 (last year for full data) and of those 77% were males. Further, males serve in the military in much larger numbers than females. According to the Department of Defense (2015), in 2014 83% of all officers in the Services (Navy, Army, Marine Corps and Air Force) were male, while 85% of all enlisted service members were male. Males are also more than three times as likely to commit suicide (CDC, 2016a).
Additionally, men are less likely than women to have health insurance, develop a regular relationship with a doctor, or seek treatment for a medical condition (Scott, 2015). Women are more religious than men, which is associated with healthier behaviors (Greenfield, Vaillant & Marks, 2009). Lastly, social contact is also important as loneliness is considered a health hazard. Nearly 20% of men over 50 have contact with their friends less than once a month, compared to only 12% of women who see friends that infrequently (Scott, 2015). Hence, men’s lower life expectancy appears to be due to both biological and lifestyle factors.
Theories of Aging
Why do we age? There are many theories that attempt to explain how we age, but researchers still do not fully understand what factors contribute to the human lifespan (Jin, 2010). Research on aging is constantly evolving and includes a variety of studies involving genetics, biochemistry, animal models, and human longitudinal studies (NIA, 2011a). According to Jin (2010), modern biological theories of human aging involve two categories. The first is Programmed Theories that follow a biological timetable, possibly a continuation of childhood development. This timetable would depend on “changes in gene expression that affect the systems responsible for maintenance, repair, and defense responses,” (p. 72). The second category includes Damage Theories which emphasize environmental factors that cause cumulative damage in organisms. Examples from each of these categories will be discussed.
Primary Aging: Programmed Theories
Genetics
Genetic make-up certainly plays a role in longevity, but scientists are still attempting to identify which genes are responsible. Based on animal models, some genes promote longer life, while other genes limit longevity.

Specifically, longevity may be due to genes that better equip someone to survive a disease. For others, some genes may accelerate the rate of aging, while others slow that rate. To help determine which genes promote longevity and how they operate, researchers scan the entire genome and compare genetic variants in those who live longer with those who have an average or shorter lifespan. For example, a National Institutes of Health study identified genes possibly associated with blood fat levels and cholesterol, both risk factors for coronary disease and early death (NIA, 2011a). Researchers believe that it is most likely a combination of many genes that affect the rate of aging.
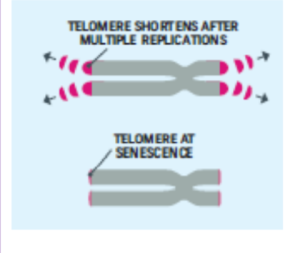
Cellular Clock Theory
This theory suggests that biological aging is due to the fact that normal cells cannot divide indefinitely. This is known as the Hayflick limit, and is evidenced in cells studied in test tubes, which divide about 40-60 times before they stop (Bartlett, 2014). But what is the mechanism behind this cellular senescence? At the end of each chromosomal strand is a sequence of DNA that does not code for any particular protein, but protects the rest of the chromosome, which is called a telomere.
With each replication, the telomere gets shorter. Once it becomes too short the cell does one of three things. It can stop replicating by turning itself off, called cellular senescence. It can stop replicating by dying, called apoptosis. Or, as in the development of cancer, it can continue to divide and become abnormal. Senescent cells can also create problems. While they may be turned off, they are not dead, thus they still interact with other cells in the body and can lead to an increase risk of disease. When we are young, senescent cells may reduce our risk of serious diseases such as cancer, but as we age they increase our risk of such problems (NIA, 2011a). The question of why cellular senescence changes from being beneficial to being detrimental is still under investigation. The answer may lead to some important clues about the aging process.
DNA Damage
Over time DNA, which contains the genetic code for all organisms, accumulates damage. This is usually not a concern as our cells are capable of repairing damage throughout our life. Further, some damage is harmless. However, some damage cannot be repaired and remains in our DNA. Scientists believe that this damage, and the body’s inability to fix itself, is an important part of aging (NIA, 2011a). As DNA damage accumulates with increasing age, it can cause cells to deteriorate and malfunction (Jin, 2010). Factors that can damage DNA include ultraviolet radiation, cigarette smoking, and exposure to hydrocarbons, such as auto exhaust and coal (Dollemore, 2006).
Mitochondrial Damage
Damage to mitochondrial DNA can lead to a decaying of the mitochondria, which is a cell organelle that uses oxygen to produce energy from food. The mitochondria convert oxygen to adenosine triphosphate (ATP) which provides the energy for the cell. When damaged, mitochondria become less efficient and generate less energy for the cell and can lead to cellular death (NIA, 2011a).
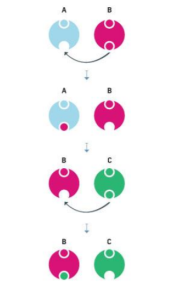
Free Radicals
When the mitochondria use oxygen to produce energy, they also produce potentially harmful byproducts called oxygen free radicals (NIA, 2011a). The free radicals are missing an electron and create instability in surrounding molecules by taking electrons from them. There is a snowball effect (A takes from B and then B takes from C, etc.) that creates more free radicals which disrupt the cell and causes it to behave abnormally (See Figure 10.22). Some free radicals are helpful as they can destroy bacteria and other harmful organisms, but for the most part they cause damage in our cells and tissue. Free radicals are identified with disorders seen in those of advanced age, including cancer, atherosclerosis, cataracts, and neurodegeneration. Some research has suggested that adding antioxidants to our diets can help counter the effects of free radical damage because the antioxidants can donate an electron that can neutralize damaged molecules. However, the research on the effectiveness of antioxidants is not conclusive (Harvard School of Public Health, 2016).

Immune and Hormonal Stress Theories
Ever notice how quickly U.S. presidents seem to age? Before and after photos reveal how stress can play a role in the aging process. When gerontologists study stress, they are not just considering major life events, such as unemployment, death of a loved one, or the birth of a child. They are also including metabolic stress, the life sustaining activities of the body, such as circulating the blood, eliminating waste, controlling body temperature, and neuronal firing in the brain. In other words, all the activities that keep the body alive also create biological stress.
To understand how this stress affects aging, researchers note that both problems with the innate and adaptive immune system play key roles. The innate immune system is made up of the skin, mucous membranes, cough reflex, stomach acid, and specialized cells that alert the body of an impending threat. With age these cells lose their ability to communicate as effectively, making it harder for the body to mobilize its defenses. The adaptive immune system includes the tonsils, spleen, bone marrow, thymus, circulatory system and the lymphatic system that work to produce and transport T cells. T-cells, or lymphocytes, fight bacteria, viruses, and other foreign threats to the body. T-cells are in a “naïve” state before they are programmed to fight an invader and become “memory cells”. These cells now remember how to fight a certain infection should the body ever come across this invader again. Memory cells can remain in your body for many decades, which is why the measles vaccine you received as a child is still protecting you from this virus today. As older adults produce fewer new T-cells to be programmed, they are less able to fight off new threats and new vaccines work less effectively. The reason why the shingles vaccine works well with older adults is because they already have some existing memory cells against the varicella virus. The shingles vaccine is acting as a booster (NIA, 2011a).
Hormonal Stress Theory, also known as Neuroendocrine Theory of Aging, suggests that as we age the ability of the hypothalamus to regulate hormones in the body begins to decline leading to metabolic problems (American Federation of Aging Research (AFAR) 2011). This decline is linked to excessive levels of the stress hormone cortisol. While many of the body’s hormones decrease with age, cortisol does not (NIH, 2014a). The more stress we experience, the more cortisol we release, and the more hypothalamic damage that occurs. Changes in hormones have been linked to several metabolic and hormone related problems that increase with age, such as diabetes (AFAR, 2011), thyroid problems (NIH, 2013), osteoporosis, and orthostatic hypotension (NIH, 2014a).
Secondary Aging: Damage Theories
A second set of theories focuses on aging as an outcome of the wear and tear our bodies receive as part of our daily lives. Damage theories examine the parts of aging and death that come from the outside. This perspective scrutinizes the nurture or environmental side of the equation, and holds that the body wears out through the cumulative effects of a host of life events and lifestyle factors, such as disease, disuse, abuse, stress, and environmental toxins. Evidence to support this position comes from three sources.
Lifestyle Factors that Predict Aging and Death
The first body of research supporting the role of nurture in aging examines the effects of a variety of physical factors, such as poor diet, lack of exercise, and substance abuse, as well as psychological factors, such as stress, social inactivity, and pessimistic outlook. Studies show that these factors can predict both how healthy people will be and how long they will live. Such forces can cause biological damage, which is repaired more and more slowly as we age. From this perspective aging results from the accumulation of such damage.
Exposure to Environmental Toxins
A second body of evidence shows that aging can be shaped by exposure to environmental pollution, as caused for example by pesticides, air pollutants, and radiation, or by harmful substances added to our food and water. When we metabolize these toxins, they do damage, not only to our bodies, but also to our our genetic DNA material at the cellular level. As our organ systems deteriorate and become more vulnerable, errors pile up- and our body’s ability to repair them slows down. Toxins can cause allergic reactions and auto-immune diseases, as seen in the upswing in Type II adult-onset diabetes and other chronic conditions. Effects of environmental toxins are also seen in inflammatory processes involved in life threatening medical conditions, like cardiovascular disease, arthritis, and cancer.
Historical Increases in Average Life Expectancy
A third source of evidence that aging can be speeded up or slowed down by external factors comes from documentation of steady historical increases in average life expectancy. These increases follow from changes in external factors, mostly related to public health. They include historical reductions in the number of people in extreme poverty as well as improvements in nutrition, quality and access to medical care, sanitation, child birth procedures, and the invention of antibiotics. These historical changes did not result in evolutionary changes in human biology. They produced recent changes in environmental factors that are shaping the rate of aging and the timing of death.
In sum, as you can see that aging and death are processes that are multiply determined– by biological, psychological, social, and contextual factors. Theories of primary aging are correct that we have the seeds of our aging programmed into our bodies. But theories of secondary aging are also correct– the environment also speeds-up and slows down our aging and dying via the damage it does to our increasingly vulnerable biology. And, of course, lifespan researchers would remind us that the active individual also plays a role through the decisions they make about behavioral risks, like smoking, drinking, and using or abusing substances, as well as via more positive routes, especially a healthy diet, exercise, and continued participation in positive social and cognitive activities.
References (Click to expand)
Licenses & Attributions (Click to expand)
CC Licensed Content
- “Lifespan Development: A Psychological Perspective, Second Edition” by Martha Lally and Suzanne Valentine-French is licensed under a CC-BY-NC-SA-3.0
- Lifespan Development by Lumen Learning is licensed under a Creative Commons Attribution 4.0 International License
- “Grief in Children and Developmental Concepts of Death” by Adam Himebauch, Robert Arnold MD, and Carol May is licensed under a CC-BY-NC-4.0
